The art of ice cream making has evolved from simple frozen desserts to sophisticated culinary creations where texture and stability are paramount. At the heart of this transformation lies the science of ice crystal nucleation control, a process critically influenced by the hydrogen bonding efficacy of stabilizers. These hydrocolloids, often derived from natural sources, serve as the unsung heroes in maintaining the creamy, smooth consistency that defines premium ice cream. Their ability to form intricate hydrogen-bonded networks with water molecules not only impedes the growth of ice crystals but also enhances the product's resistance to thermal shock and extends its shelf life. As consumer demand for high-quality, natural, and stable frozen desserts grows, understanding the molecular interactions of these stabilizers becomes increasingly vital for both artisanal producers and industrial manufacturers.
Hydrogen bonding represents one of the most fundamental intermolecular forces in nature, playing a crucial role in the structural integrity of countless biological and synthetic systems. In the context of ice cream, water molecules naturally form hydrogen bonds to create ice crystals during the freezing process. Without intervention, these crystals can grow large enough to be detected by the human palate, resulting in a coarse, icy texture. Stabilizers function by competing with this natural crystallization process. Their hydroxyl-rich molecular structures allow them to form extensive hydrogen bonds with water, effectively reducing the amount of free water available for ice crystal formation and growth. This molecular interference creates a more controlled freezing environment, leading to the formation of numerous, but exceedingly small, ice crystals that contribute to a smooth mouthfeel.
The efficacy of a stabilizer is directly tied to its hydrogen bonding capacity, which varies significantly across different types of hydrocolloids. Common stabilizers like guar gum, locust bean gum, carrageenan, and xanthan gum each possess unique molecular architectures that dictate their interaction with water. Guar gum, for instance, features a high density of hydroxyl groups along its galactomannan chain, enabling it to form a vast network of hydrogen bonds that effectively binds water and increases viscosity. Locust bean gum requires heat activation to achieve its full hydrogen bonding potential, after which it forms particularly strong gels that synergize well with other stabilizers. Carrageenan's sulfated galactose units allow for both hydrogen bonding and ionic interactions, making it exceptionally effective in dairy systems where it interacts with casein proteins. Xanthan gum's rigid helical structure stabilized by hydrogen bonds provides remarkable stability across a wide range of temperatures and pH levels.
In commercial ice cream production, stabilizer blends rather than single hydrocolloids are typically employed to leverage synergistic hydrogen bonding effects. These carefully formulated combinations create a more robust network than any single stabilizer could achieve alone. For example, the combination of locust bean gum and carrageenan creates a gel network where each polymer contributes distinct hydrogen bonding characteristics, resulting in superior melt resistance and heat shock protection. The development of these blends requires deep understanding of each component's hydrogen bonding behavior under different conditions of temperature, concentration, and pH. Modern ice cream stabilizer systems often incorporate 3-5 different hydrocolloids, each selected for their specific contribution to the overall hydrogen bonding matrix that controls ice crystal size distribution.
The process of hydrogen bond formation between stabilizers and water molecules occurs throughout the manufacturing process, with critical phases during mixing, pasteurization, aging, and freezing. During pasteurization, heat energy increases molecular mobility, allowing stabilizers to fully hydrate and establish their hydrogen bonding networks. The subsequent aging period, typically lasting 4-24 hours at refrigeration temperatures, allows these networks to mature and strengthen. This aging process is crucial for developing the viscosity and water-binding capacity that will control ice crystal size during dynamic freezing. In the freezer, as water begins to crystallize, the pre-established hydrogen bonded network physically obstructs crystal growth and recruits water molecules that might otherwise join growing ice crystals.
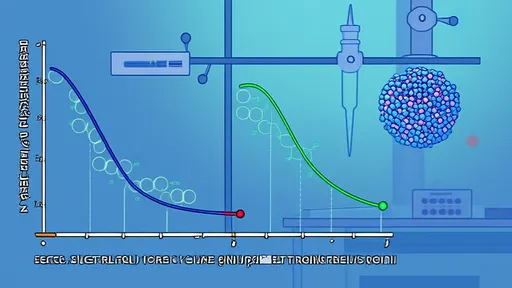
Advanced analytical techniques have revolutionized our understanding of stabilizer-water interactions at the molecular level. Nuclear Magnetic Resonance (NMR) spectroscopy allows researchers to quantify the strength and quantity of hydrogen bonds formed between specific stabilizers and water molecules. Differential Scanning Calorimetry (DSC) measures how these bonds affect the freezing and melting behavior of water in the ice cream mix. Cryo-Scanning Electron Microscopy (Cryo-SEM) provides visual evidence of how the hydrogen-bonded network influences ice crystal architecture. These tools have revealed that effective stabilizers don't prevent ice crystal formation entirely but rather create a controlled environment where crystals nucleate in greater numbers but remain microscopic in size, typically below 50 micrometers, which is the detection threshold of the human tongue.
The hydrogen bonding efficacy of stabilizers directly impacts several critical quality parameters in finished ice cream. Products with optimized stabilizer systems demonstrate significantly reduced ice crystal growth during temperature fluctuations, a phenomenon known as heat shock. This stability translates to extended shelf life without degradation of texture. The melt-down characteristics are also profoundly influenced, with properly formulated hydrogen bonding networks ensuring that ice cream melts slowly and evenly, rather than separating into watery liquid and foam. The mouthfeel benefits from the minute ice crystal size, perceived as exceptional smoothness rather than iciness. Additionally, the hydrogen-bonded water contributes to enhanced flavor release, as the stabilized water phase better carries and delivers flavor compounds to the taste receptors.
As consumer preferences shift toward cleaner labels and natural ingredients, the ice cream industry faces new challenges in ice crystal control. Traditional stabilizers, while effective, often appear as unfamiliar chemical names on ingredient statements. This has driven research into alternative hydrocolloids from recognizable sources like citrus fiber, apple pectin, and various starches that can form effective hydrogen bonding networks. These natural alternatives must achieve similar hydrogen bonding efficacy to their synthetic counterparts while meeting consumer expectations for simplicity and familiarity. The development of such systems requires sophisticated understanding of how processing conditions affect the hydrogen bonding capacity of these natural polymers and how they interact with other components in the ice cream matrix.
Future innovations in ice cream stabilization will likely focus on enhancing the precision of hydrogen bond management. This may include the development of novel stabilizers with optimized molecular structures for maximum hydrogen bonding efficiency, possibly through enzymatic modification of natural polymers. Smart stabilizer systems that respond to temperature changes by altering their hydrogen bonding patterns could provide even greater protection against heat shock. Nanotechnology approaches might enable the creation of stabilizer particles with precisely engineered surfaces designed for optimal water interaction. As our fundamental understanding of molecular interactions in frozen systems deepens, the potential for creating ice creams with unprecedented stability and texture continues to expand, all rooted in the sophisticated management of hydrogen bonds between stabilizers and water molecules.
The control of ice crystal nucleation through hydrogen bonding represents a perfect marriage of food science and culinary art. While consumers enjoy the sensory pleasure of smooth, creamy ice cream, food scientists appreciate the complex molecular dance occurring between stabilizers and water molecules that makes this experience possible. The continued refinement of stabilizer systems based on hydrogen bonding principles ensures that ice cream can maintain its quality from production through distribution to final consumption, regardless of temperature challenges encountered along the way. This scientific understanding empowers manufacturers to deliver consistently excellent products while providing opportunities for innovation in texture, stability, and ingredient profiles that meet evolving consumer demands.

By /Aug 29, 2025
By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025
By /Aug 29, 2025
By /Aug 29, 2025
By /Aug 29, 2025
By /Aug 29, 2025
By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025