The world of food science continually reveals the intricate dance between nature's building blocks and human ingenuity. Among these fascinating interactions, the formation of three-dimensional networks through pectin-calcium ion cross-linking stands as a cornerstone of modern food texture engineering. This process, seemingly simple in concept yet profoundly complex in execution, governs the structural integrity of countless food products, from the delicate set of a fruit jelly to the firm bite of a sugar-reduced confection. It represents a perfect marriage between a natural polysaccharide and a mineral ion, creating structures that are both scientifically elegant and commercially vital.
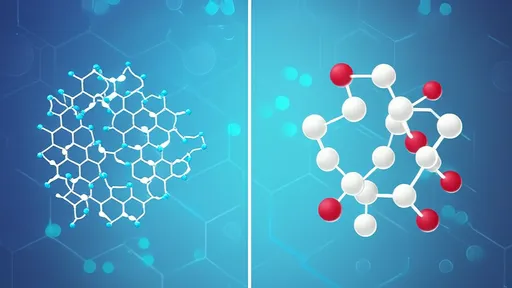
Pectin, a complex heteropolysaccharide predominantly found in the cell walls of terrestrial plants, serves as the foundational polymer in this structural symphony. Its molecular structure, characterized by a backbone of α-(1-4)-linked D-galacturonic acid units, creates opportunities for specific interactions that define texture. The degree of methoxylation—the proportion of galacturonic acid units esterified with methanol—creates what scientists categorize as high-methoxyl (HM) and low-methoxyl (LM) pectins. This distinction proves crucial, as LM pectins, with their lower degree of esterification, possess more free carboxyl groups that become actively involved in what is known as the "egg-box" model of cross-linking.
The magic begins when these LM pectin molecules encounter calcium ions in solution. The calcium ions, carrying a positive charge, interact with the negatively charged carboxyl groups on the pectin chains. This interaction doesn't occur randomly; rather, it follows a specific molecular recognition pattern. Sections of the pectin chain rich in consecutive de-esterified galacturonic acid residues form organized stretches that can accommodate calcium ions in a coordinated manner. The resulting structure resembles eggs neatly arranged in an egg box—hence the name given to this model by food scientists.
This egg-box formation doesn't happen instantaneously nor does it occur in isolation. The process unfolds through several stages, beginning with the initial binding of calcium ions to individual pectin chains. These bound ions then serve as junction zones for other pectin molecules, gradually building a continuous three-dimensional network throughout the solution. The strength and characteristics of this network depend profoundly on multiple factors: the concentration of both pectin and calcium ions, the pH of the system, the temperature during gelation, and the precise molecular weight and degree of esterification of the pectin used.
Food technologists have learned to manipulate these variables to achieve specific textural outcomes. For instance, a higher calcium concentration typically leads to a firmer, more brittle gel structure, while lower concentrations yield softer, more spreadable textures. The pH plays a critical role too, as it affects the charge density on the pectin molecules. At lower pH values, closer to the pKa of galacturonic acid (approximately 3.5), fewer carboxyl groups are ionized, reducing the potential for calcium cross-linking. This knowledge allows manufacturers to precisely engineer products with exactly the right mouthfeel and structural stability.
Beyond texture modification, the pectin-calcium system offers significant nutritional advantages. As the food industry seeks to reduce sugar content in response to consumer health demands, LM pectin-calcium gels provide an invaluable tool. Unlike traditional HM pectin gels that require high sugar concentrations (typically 55-85% soluble solids) and low pH to form, LM pectin gels can set with little to no added sugar. This capability has revolutionized product development for diabetic-friendly, reduced-sugar, and "no added sugar" product categories without compromising the enjoyable texture consumers expect.
The applications extend far beyond conventional jams and jellies. Innovative chefs and food developers now use pectin-calcium systems to create fluid gels, spherifications, and other molecular gastronomy techniques that delight modern consumers. In the pharmaceutical industry, this same cross-linking mechanism enables the development of controlled-release drug delivery systems and protective encapsulation of sensitive bioactive compounds. The beauty of this system lies in its food-grade status and natural origin, making it acceptable to consumers seeking clean-label ingredients.
Recent research continues to push the boundaries of what's possible with pectin-calcium networks. Scientists are exploring modified pectins with specific block-wise patterns of esterification to create even more precise gelation behaviors. Others are investigating the combination of pectin with other polysaccharides like alginate or chitosan to create composite gels with enhanced functional properties. The interaction between pectin-calcium gels and other food components, such as proteins and starches, represents another frontier in understanding how to build better food structures.
Despite the advanced understanding of the egg-box model, mysteries remain. The kinetics of network formation—exactly how and at what rate the junctions form—continues to be an active area of research. Advanced analytical techniques including atomic force microscopy, small-angle X-ray scattering, and rheological modeling are providing new insights into the nanoscale organization of these gels. Each discovery opens new possibilities for applications not just in food, but in biomedicine, environmental science, and materials engineering.
As we look to the future of food processing, the humble partnership between pectin and calcium ions stands as a testament to how deeply understanding fundamental scientific principles can drive innovation. What begins as a simple interaction between a plant polysaccharide and a mineral ion becomes the foundation for creating diverse textures, reducing sugar content, developing novel food experiences, and potentially addressing broader challenges in materials science. This ongoing exploration of nature's building blocks continues to reveal that sometimes, the most profound solutions come from understanding the simplest interactions.

By /Aug 29, 2025
By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025
By /Aug 29, 2025
By /Aug 29, 2025
By /Aug 29, 2025
By /Aug 29, 2025
By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025