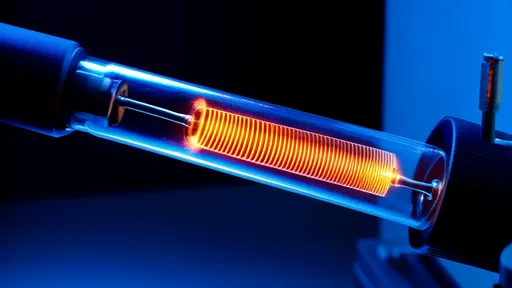
The ethereal glow of neon gas has captivated scientists and artists alike for over a century, yet its underlying quantum mechanical dance remains one of the most exquisite examples of nature's subtle artistry. Within the confines of a discharge tube, a silent symphony of quantum transitions unfolds, each photon emitted a testament to the precise and probabilistic laws that govern atomic behavior. The study of neon's glow discharge is not merely an academic exercise; it is a window into the fundamental processes that light up our universe, from the humble neon sign to the vast, glowing nebulae light-years away.
At the heart of neon's luminescence lies the process of electrical discharge. When a high voltage is applied across a sealed tube containing neon gas at low pressure, free electrons are accelerated by the electric field, hurtling through the atomic landscape with increasing energy. These electrons, acting as microscopic projectiles, collide with neutral neon atoms in their path. Most collisions are gentle brushes, but some are violent enough to disrupt the atom's delicate electronic structure, either ionizing the atom or elevating one of its electrons to a higher energy level—an excited state. This initial energy transfer sets the stage for the quantum performance to follow.
The neon atom, with its ten electrons, possesses a complex and precisely ordered set of energy levels. The outermost electrons, the valence electrons, reside in the 2p orbital in the ground state. Upon collision, an electron may be excited to a higher orbital, such as the 3s, 3p, 4s, or even higher states. However, these excited states are inherently unstable. Like a ball perched on a steep hill, the electron yearns to return to a lower, more stable energy configuration. It cannot simply slide down; it must make a discrete jump, a quantum leap, shedding its excess energy in the form of a photon. The energy of this photon, and thus its wavelength and color, is precisely determined by the difference between these two quantized energy levels: E_photon = E_upper - E_lower.
This is where neon's signature crimson-orange glow originates. The most prominent and probable transitions for neon occur when an electron falls from a 3p orbital down to a 3s orbital. The specific energy difference for these transitions corresponds to photons with wavelengths around 640 nanometers, which the human eye perceives as a vibrant red-orange light. This is not a single, monochromatic light, but a blend of several very close spectral lines, giving the glow its rich and characteristic hue. Other transitions, such as those to the 2p level, produce photons in the infrared and ultraviolet ranges, invisible to us but no less real, completing the atom's full spectral fingerprint.
The environment within the discharge tube is a chaotic plasma of ions, free electrons, and neutral atoms, all in constant motion. This chaos is elegantly organized by collision processes. An excited neon atom might not always radiate its energy immediately. It can transfer its excitation energy to another atom through a second collision in a process known as collisional de-excitation, or the energy can be transferred in a resonant energy transfer if it encounters an atom of the same species. Furthermore, the free electrons themselves are not all identical; they possess a distribution of energies. The glow discharge's appearance—its color intensity and distribution between the cathode and anode—is directly dictated by this electron energy distribution and the specific rates of these countless collision processes.
Observing the glow reveals a fascinating structure. It is not a uniform field of light. Directly adjacent to the cathode is a thin, dim layer known as the Aston Dark Space. Next comes the Cathode Glow, a luminous region followed by another dark zone, the Cathode Dark Space or Hittorf's Dark Space. This is followed by the most prominent feature: the negative glow, a bright, diffuse light that extends into the positive column, the long, glowing region that most people associate with a neon light. Each of these regions corresponds to a specific set of dominant physical processes. The negative glow, for instance, is where electron energies are optimal for exciting neon atoms to the states that yield the famous red light. The positive column is a region of lower field strength where electrons and ions recombine and lower-energy excitations occur.
The quantum mechanics of these transitions are described by transition probabilities and selection rules. Not every conceivable jump between energy levels is allowed. Quantum selection rules, based on changes in angular momentum quantum numbers, dictate which transitions are "allowed" and which are "forbidden." The strongest lines in the neon spectrum are those with the highest transition probabilities, or oscillator strengths. Forbidden lines, while possible under low-pressure conditions over long timescales, are vastly weaker. The brilliant red glow we see is a direct consequence of these rules, a physical manifestation of the mathematical symmetries embedded in the quantum world.
Understanding neon's glow discharge has profound implications far beyond aesthetic appeal. It serves as a fundamental benchmark in atomic physics for calibrating spectroscopic equipment. The well-known and stable spectral lines of neon are used as a ruler to measure the light from other, unknown sources. The principles learned from neon discharges are directly applicable to the development of gas lasers, such as the ubiquitous helium-neon laser, where the neon atom is the active lasing medium, its population inversions created through precise energy transfer from excited helium atoms. Furthermore, the study of plasmas in neon provides a manageable model for understanding more complex plasma behavior in fields ranging from industrial processing to astrophysics, helping scientists decipher the light from distant stars and interstellar clouds.
In conclusion, the radiant glow of a neon tube is a masterpiece of quantum physics in action. It is a dynamic, bustling environment where kinetic energy is converted into light through a meticulously choreographed series of collisions and quantum jumps. Each pulse of crimson light is a story—a narrative of acceleration, impact, excitation, and a final, luminous leap back home. It reminds us that the universe's most spectacular displays are often the result of its smallest, most precise, and deeply fundamental laws. The neon sign is more than commercial signage; it is a beacon of human understanding, illuminating the intricate and beautiful workings of the atomic realm.

By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025
By /Aug 27, 2025
By /Aug 27, 2025

By /Aug 27, 2025

By /Aug 27, 2025